Women with dense breast tissue should get regular screenings: FDA
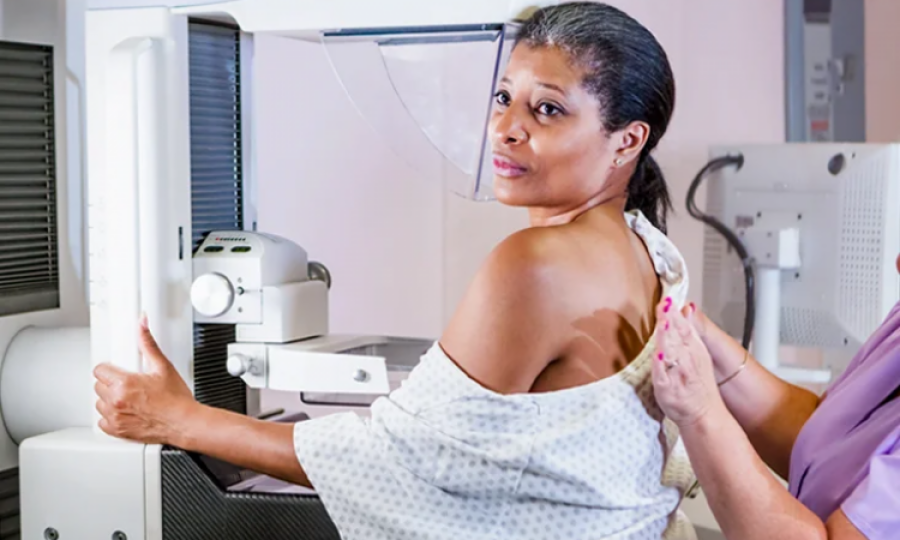
USA: Recently, Jounarlist Katie Couric revealed that she had been diagnosed, and treated breast cancer. She advised that women with denser breast tissue, like herself, should get regular ultrasounds or MRIs to detect breast cancer since denser breast tissue has a tendency to conceal cancer lesions and can become difficult to detect through radiography. The FDA seeks to forward the passing for a legislation on breast density notification.
Since 2019, the USA's Food and Drug Administration has been seeking to send out a state notification regarding breast density. However, there have been conflicting opinions on supplemental screening. Advocates believe that it could help disease diagnosis through early screening and that literature on the breast density and its association to cancer is limited. There have also been claims that women across several cultures, ethnicities and literacy levels have had conflicting opinions and reaction to the state's legislations.
In order for the state to send out notification that women acorss the country can understand and implement, the state will have to look into the its mode of delivery and language.
Based on substantial evidence, women’s responses and reactions to states’ previous breast density notifications, have been negative. Indeed, some women misunderstand the meaning of breast density and its effect on cancer detection. Many in the medical, research, and advocacy communities provided comments on the proposed FDA language in 2019, identifying several instances where the quality of communication requires improvement. However, the extent to which feedback has been used to inform the final notification has not been made public.
Advertisement
Trending
Popular
Hair loss: Discovery uncovers key stem cells that could reverse ...
-
Broccoli sprout compound may help lower ...
11:31 AM, 25 Feb, 2025 -
Gas Pain vs. Heart Attack: How to tell ...
09:00 PM, 22 Feb, 2025 -
Coconut oil supplement shows promise ...
08:00 PM, 20 Feb, 2025 -
Normal vitamin B12 levels may still ...
05:00 PM, 19 Feb, 2025



